Abstract
Background: The efficacy and safety of rivaroxaban versus enoxaparin/vitamin K antagonist (VKA) for treatment and secondary prevention of venous thromboembolism (VTE) was demonstrated in the EINSTEIN clinical trial program. As screening for primary hypercoagulable states was not required as part of the EINSTEIN trial program protocol, fewer than 500 total patients with a known primary hypercoagulable state were identified.
Objective: To assess the effectiveness and safety of rivaroxaban versus warfarin for treatment and secondary prevention of VTE in patients with a known primary hypercoagulable state in a large, real-world population.
Methods: US MarketScan claims data from January 2012-September 2015 were utilized. We identified adult patients with a primary International Classification of Diseases-9th Revision (ICD-9) discharge diagnosis code for deep vein thrombosis (DVT) (ICD-9=451.1, 451.2, 453.40, 453.41, 453.42, 453.8, 453.9) or pulmonary embolism (PE)(ICD-9=415.1x), diagnosed during a hospitalization or emergency department visit (the index event), with ≥180-days of continuous medical and prescription benefits prior to the index event (baseline), a documented diagnosis for a primary hypercoagulable state (ICD-9=289.81) during baseline or the index VTE encounter and newly-initiated on rivaroxaban or warfarin within 30-days of the index VTE event. Patients with a claim for an anticoagulant before the index event were excluded. Effectiveness and safety endpoints included recurrent VTE, any major bleeding (identified per the Cunningham algorithm), intracranial hemorrhage (ICH) and gastrointestinal bleeding (GIB). Patients were followed for a maximum of 12-months from the index event or until occurrence of an endpoint, switch or discontinuation (14-day permissible gap) of index oral anticoagulation or insurance disenrollment. Rivaroxaban users were 1:1 propensity-score matched to warfarin users. Balance between cohorts was evaluated by inspecting standardized differences for baseline covariates (differences >0.1 indicating imbalance). Cox regression was performed and results reported as hazard ratios (HRs) with 95% confidence intervals (CIs).
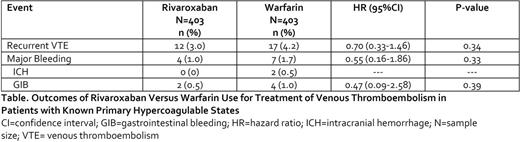
Results: We matched 403 rivaroxaban and 403 warfarin users with a known primary hypercoagulable state and experiencing a VTE. Mean±standard deviation (SD) duration of patient follow-up was 0.5±3 years. All baseline covariates had a standardized difference <0.1 after matching. Mean±SD age of the population was 50±14 years; PE (with or without DVT) was present in 53% of patients, 50% of patients were female and 12% had a hospital admission within 180-days of their qualifying VTE event. Rivaroxaban use was associated with a 30% non-significant relative reduction in the hazard of recurrent VTE and a non-significant 45% relative reduction in the hazard of major bleeding as compared to warfarin (Table). Only 2 patients experienced an ICH (both warfarin users). Rivaroxaban users had a non-significant 53% relative reduction in the hazard of GIB versus warfarin.
Conclusion: Our study results suggest rivaroxaban's relative effectiveness and safety versus warfarin is maintained when treating VTE patients with known primary hypercoagulable states in routine practice. These real-world study findings are consistent with that of the overall EINSTEIN clinical trial program and its subgroup analysis of patients with primary hypercoagulable states.
Coleman: Boehringer Ingelheim: Consultancy, Honoraria; Janssen Pharmaceuticals: Consultancy, Honoraria, Research Funding; Bayer AG: Consultancy, Honoraria, Research Funding. Turpie: Bayer HealthCare Pharmaceuticals: Consultancy; Janssen Research and Development: Consultancy, Honoraria. Beyer-Westendorf: Pfizer: Honoraria, Research Funding; Daiichi-Sankyo: Honoraria, Research Funding; Bayer: Honoraria, Research Funding; Boehringer-Ingelheim: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal